For almost 50 years, Spami has been the technology leader in designing and manufacturing automated lines for the transformation of glass tubing into containers for pharmaceutical use.
As part of Stevanato Group, Spami also draws on a wealth of technology capabilities in related areas.
 |  |
KEY STRONG POINTS
Spami’s main strengths are its technological leadership in automated lines, combined with its in-house knowledge and manufacturing capabilities. Because it is part of the wider Stevanato Group, Spami not only supplies automated lines, it has also gained in-depth understanding of the pharmaceutical glass primary packaging market.
Spami continuously adapts its technology, addressing the real and changing needs of pharmaceutical glass container manufacturers while offering higher quality, higher process stability, faster output and greater ease of use. “Our customers are very demanding and we continually adapt our forming technologies to market requirements,” says Paolo Ziero, manager of the Glass Forming business unit.
Where others only sell single parts of a production line or combine machinery from different suppliers, Spami is a true ‘one-stop shop’ provider, which can offer glass forming lines as stand-alone models or as entire lines. “Everything we do is made in-house, thanks to our skilled and passionate engineers and workshop team. Our equipment provides the most accurate processing of ampoules, vials, cartridges and syringes to be found in the industry. We achieve this through delicate container handling throughout the forming process and rigorous container inspection via state-of-the-art camera control systems,” adds Ziero.
GLASS CONVERTING LINES
Spami manufacturing forming lines in a variety of specifications depending on the container (ampoules, vials, cartridges, syringes) and the specific production requirements.
By maintaining close ties with the customer throughout the process, we can design and manufacture fully tailored, purpose-built equipment capable of producing containers with entirely customised dimensions and shapes. The key attributes of our glass converting lines are:
- optimal mechanical durability, thanks to carefully selected raw materials and an extremely accurate assembly process;
- continuous monitoring of all critical parameters that influence the forming process;
- high mechanical precision and easy-to-use HMIs, ensuring seamless high-speed production, fewer rejects and mechanical resistance;
- cutting-edge, easy-to-use, automatic production lines to maximise production efficiency, preventing human error and obtaining premium quality glass containers.
Our converting lines come as a turnkey package, including the forming unit and other process equipment, or as stand-alone modules. Completely automatic washing equipment is also available to remove particles from the glass and reduce cosmetic defects.
MODULES IN CONVERTING LINES
The different modules on offer begin with tube loaders. Spami has developed an automatic loader that allows glass tubing to be fed automatically into vertical rotating machines. This reduces the number of personnel required, as the operator only needs to load one or more bundles from a safe and ergonomic position.
The automatic tube loader is equipped with a servo motor to move the mechanical arm. Thus, the movement can be synchronised with the converting line, providing a seamless production process for vials, cartridges, syringes and ampoules. This technology also improves process control and reduces maintenance requirements. The loader is available in a ‘no glass-to-glass’ version as well.
Our after-forming lines ensure the highest stability during transport to the annealing oven. The special material used for conveying preserves the cosmetic quality of the glass and its mechanical features. Each line is equipped with a cooling system and dimensional control devices. Additional devices are available on request.
There are two transport options: chain transport for vials, cartridges, syringes and ampoules, in which the containers move horizontally along a chain; and prism transport, in which vials and cartridges move horizontally along a prismatic line. Both include a contact point made of a heat-resistant plastic material to guarantee container quality and no glass-to-metal or glass-to-glass contact.
Spami offers two annealing furnace models, with either gas burners or electric heating elements, for the annealing of ampoules, vials, cartridges and syringes. These machines are designed to provide an optimal thermal profile and are cost-convenient, high-performance and safe to operate.
Both types of furnace are built around solid cross-section bars made of welded steel. The belt is made of stainless steel in order to resist the high temperatures reached inside the chamber. Heat is maintained by the use of refractory and insulating materials, which also ensures energy saving and safety for users.
The tunnel is equipped with thermocouples to monitor the thermal cycle, while the self-adjusting system helps to keep the temperature within the tolerances set by the operator. Other key features include no glass-to-glass contact, automatic control of the thermal cycle, belt speed control and high annealing uniformity in vials, thanks to the containers’ vertical position.
The final module comprises an automatic packing machine. This is designed to transfer the containers gently from the annealing oven to trays or blisters, minimising the need for human operators and reducing the risk of contamination or human error. A five-position rotary table acts as a buffer and further adds to the system’s autonomy.
The packing machine can be equipped with an automatic cosmetic control system to maintain control until the very end of the process. The line is extremely flexible, requiring only a few format change-over parts, and it is also possible to integrate further added-value components into it.
The machine can be equipped with a chain conveyor system or a roller conveyor to support the cosmetic inspection system and the box dimensions can be customised, based on the customer’s specific needs. A dedicated station to control printing can also be installed at this stage of the process.
SILICONISATION
Another key operation on our glass vial and syringe forming lines, significantly influencing container performance, is siliconisation. Silicone distribution must be optimised and monitored to ensure safe and effective administration to the patient, and to guarantee drug quality.
Among other things, silicone in the form of an emulsion and/or an oil, minimises interaction between the product and the container and prevents certain products from sticking to the internal surface, making the glass appear perfectly transparent.
Spami’s siliconisation lines are specifically designed to:
- increase gliding performance between the glass and internal components (e.g. rubber parts);
- improve the flow of liquid solutions inside the container, reducing ‘dead volume’ concerns; and
- reduce interactions between glass and the product.
DIMENSIONAL AND COSMETIC CONTROLS
Extensive inline controls, both dimensional and cosmetic, to ensure the correct dimensions and appearance in the containers, are a particular highlight of Spami’s capabilities. These are increasingly in demand from pharmaceutical companies, who have very tight quality requirements.
“Our systems use automatic camera inspection to ensure optimal setting-up of the machine and monitor the efficiency of the process. Thanks to our deeply rooted connection with the pharmaceutical industry, we develop and manufacture control systems satisfying the most demanding requirements,” says Paolo Ziero.
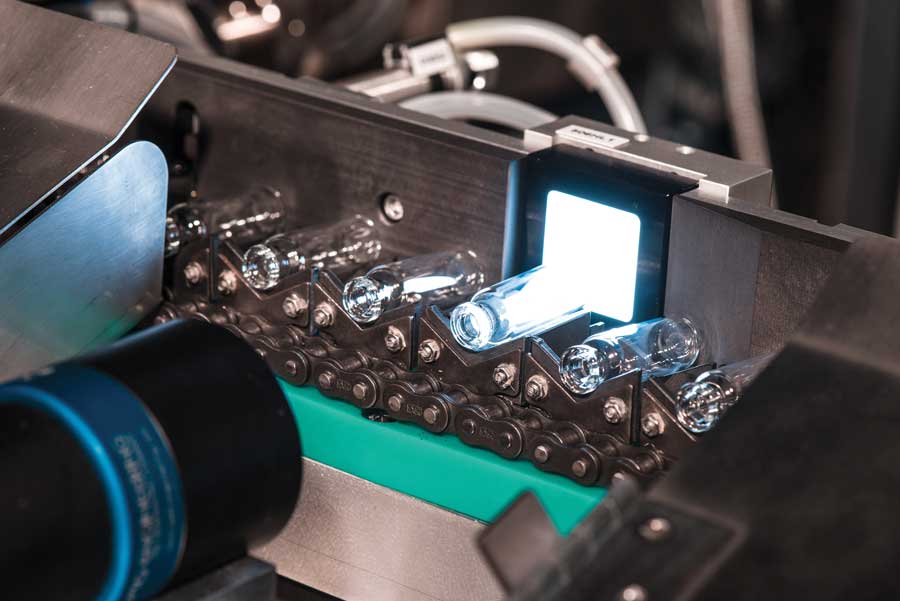
Spami’s dimensional control systems measure all of the dimensions of a vial, syringe, cartridge or ampoule. Lines can be equipped with multiple cameras depending on the customer’s requirements and the machine’s model. These cameras are positioned to inspect the mouth and shoulder areas of vials and cartridges. For syringes, different cameras are designed to inspect the cone and the flange.
The post-forming line features electromechanical gauges designed to measure the overall length of the container, the concavity of the bottom for vials and the flange thickness in syringes. Additional cameras can be added to check the inner diameter of the mouth and cone. Total length and the bottom concavity controls via cameras have also been implemented, thus avoiding metal-to-glass contact.
Spami’s inline cosmetic controls make it possible to detect any cosmetic defects, including contamination, scratches, deformations, bubbles, pressure marks, hairlines, chips and cracks. These inspection systems are installed on the conveyor line between the annealing oven and the packing unit.
Different configurations are available based on the container and customer requirements and up to nine cameras can be installed for 360° cosmetic inspection of glass containers.
Among other advantages, Spami’s inline control systems enable:
- control of all dimensions;
- multiple areas of inspection;
- customisable quality levels;
- automatic rejection of defective products;
- digitally stored measurements;
- statistical analysis of each control/parameter;
- graphic display of each parameter;
- real-time production data on HMI;
- automatic sampling and calibration systems; and
- the ability to set custom recipes.
Last but not least, to meet converting companies’ needs, Spami has designed EVA, an off-line machine capable of processing and inspecting vials from multiple lines simultaneously at speeds of up to 12,000 items per hour. It is equipped with a high-performance camera inspection
system that detects cosmetic defects along the entire surface of the vials.
EVA is extremely flexible and can cover the full range of standard vials. Containers can be loaded and unloaded onto trays or a conveyor and it can be equipped with an additional station for dimensional inspection.
READY-TO-USE CONTAINERS
Leveraging its expertise in the pharmaceutical industry, Spami designs and manufactures high-yield, highly automated equipment for primary packaging production lines in an aseptic environment.
Ready-to-use containers are a special class of primary packaging that can be filled directly, without any intermediate process, since the products and the related packaging are provided in sterile form, ready for integration into the filling machines.
To fulfil this requirement, a specific sequence of processes must be applied that can be combined to reach different product specifications on the various types of primary packaging containers (vial, cartridge, syringe, or hybrid). Spami offers flexible production lines, including nest-&-tub, Tyvek sheet and lead sealing and bagging modules, which are able to process all formats of ready-to-fill glass containers.
The main advantages are:
- no glass-to-glass loading and unloading;
- no format change needed;
- 100% inline dimensional and cosmetic controls;
- seamless integration with all washing machines and depyrogenation tunnels available on the market.

SAFETY MEASURES
Uniquely in the industry, Spami’s systems are FULL CE-compliant. In Europe, this includes EU Directives 2006/42/EC, the Machinery Directive; 2014/35/EU ‘On the safety of low-tension apparatus; and 2013/30/EU ‘On electromagnetic compatibility’. They also comply with North American standards, as laid down by ANSI, UL and NFPA, and with Russian and Brazilian standards.
“Since 2014, we have entered into a more active phase of compliance, including:
- integrating safety into the beginning design process phase;
- adding in the possibility of scheduling interventions;
- enhancement of product documentation with a modular approach by machine family;
- a higher level of procedures and documentation;
- greater recognition of technical solutions by operators, with less impact on OEE,” says Ziero.
Multiple further actions have recently taken place in the areas of documentation, validation, security and training. Spami has also boosted its capacity for analysis, validation and documentation, thus reducing costs, intervention times.
The company’s approach to safety is three-fold:
1. Project definition: Spami works proactively, by including safety evaluations when designing equipment and carrying out accurate risk analysis.
2. Risk management: Spami provides extensive documentation on how to handle equipment, reduce risks and integrate solutions that enable operators to work without risk of injury (i.e. remote control, electrical access to the machine, etc.).
3. Operator safety: Spami ensures operators can work safely, thus reducing the risk of accidents.
None of these safety measures impact the OEE of the machinery, however. Productivity remains high when they are in place.
CONCLUSION
Spami is the number one choice when it comes to forming lines. The company not only has 50 years of experience, but also draws on 70+ years of experience in pharmaceutical glass primary packaging and a wide range of related capabilities within Stevanato Group.
Uniquely among players in the market today, Spami is a true one-stop-shop, offering both complete forming lines and individual modules, all of which are designed and manufactured in-house. Whether it is ampoules, vials, cartridges and syringes, Spami’s systems provide the most accurate processing available. As well as an industry-leading range of different modules, Spami offers everything necessary for the production of ready-to-use containers in aseptic environment. Moreover, Spami is the only company that offers full CE-compliance, within a culture of innovation, rigorous standards, continuous improvement and rapid response to market needs.

Spami
www.stevanatogroup.com